m-s-m cationic gemini and zwitterionic surfactants – a thermodynamic analysis of their mixed micelle formation
Abstract
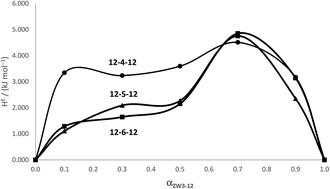
Micelle formation enthalpies (ΔmicH values) have been calorimetrically determined at 298 K for three sets of mixed zwitterionic/cationic gemini systems consisting of N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (ZW3-12) and a series of structurally related gemini surfactants, the N,N'-bis(dimethyldodecyl)-α,ω-alkanediammonium dibromide (12-s-12) systems. From the experimental and the estimated ideal micelle formation enthalpies, the excess enthalpies were obtained. The degrees of nonideality of the interaction in the mixed micelle (βm) from our previous work was used along with the excess enthalpy values to determine excess thermodynamic quantities of the surfactants in the mixed system according to Regular Solution Theory (RST) and Motomura's theory. The excess enthalpies for the ZW3-12/12-4-12 were positive in magnitude and rose sharply when small amounts of the zwittergent were distributed into the gemini micelles. The excess enthalpies for the ZW3-12/12-5-12 and the ZW3-12/12-6-12 systems were also >0 kJ mol−1, and as a function of zwittergent composition, were quite different to those of the ZW3-12/12-4-12 mixed micelles. These results indicate that the heat of mixed micelle formation is strongly dependent on electrostatic interactions and the structure of the surfactants involved, specifically, the length of the tether group for the 12-s-12 gemini surfactants. From the calorimetric data and the application of RST and Motomura's theory, we have obtained estimates of the excess Gibbs energy and entropy of mixing. An analysis of the three thermodynamic properties suggests that the relative contributions of enthalpic and entropic effects to nonideal behavior for mixed micelles involving gemini surfactants are strongly dependent on the gemini structure.
- This article is part of the themed collection: 2020 RSC Advances HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...