Room-temperature graphitization in a solid-phase reaction
Abstract
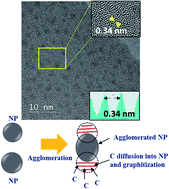
Graphitized carbon including graphene has recently become one of the most investigated advanced materials for future device applications, but a prerequisite for broadening its range of applications is to lower its growth temperature. Here we report a great decrease in graphitization temperature using the well-known catalyst Ni. Amorphous carbon films with Ni nanoparticles (NPs) were deposited, using a simple one-step magnetron sputtering method, onto microgrids and a SiO2/Si substrate for transmission electron microscopy (TEM) and Raman spectroscopy analyses, respectively. The amorphous carbon surroundings and locations between the Ni NPs started to become graphitized during the film deposition even at room temperature (RT) and 50 °C. The graphitization was confirmed by both high-resolution TEM (HR-TEM) and Raman 2D peak analyses. The increase in the relative amount of Ni in the amorphous carbon film led to the partial oxidation of the larger Ni NPs, resulting in less graphitization even at an elevated deposition temperature. Based on the detailed HR-TEM analyses, a decreased oxidation of NPs and enhanced solubility of carbon into Ni NPs were believed to be key for achieving low-temperature graphitization.


 Please wait while we load your content...
Please wait while we load your content...