Palladium-catalyzed carbonylations of highly substituted olefins using CO-surrogates†
Abstract
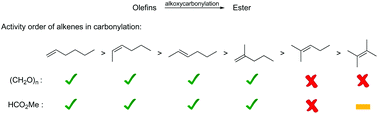
The first active catalyst system for the carbonylation of tri- and tetra-substituted olefins with paraformaldehyde and methyl formate is described. Specifically, using 1.0 mol% Pd(OAc)2 and 4.0 mol% 1,2-bis-(tert-butylpyridylphosphino)xylene L3 in the presence of 5.0 mol% PTSA·H2O, and 0.5 mol% Pd(OAc)2 and 2.0 mol% L3 in the presence of 8.0 mol% PTSA·H2O, respectively, allowed the corresponding linear esters to be obtained in good yields and selectivities.


 Please wait while we load your content...
Please wait while we load your content...