Functionalisation of esters via 1,3-chelation using NaOtBu: mechanistic investigations and synthetic applications†
Abstract
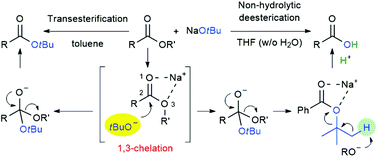
For the first time, both 1,3-chelation and the formation of a tetrahedral intermediate were confirmed as the key factors for the unusual nucleophilic behavior of a metal t-butoxide in a transesterification reaction. NMR and real-time IR spectroscopies and deuterium-labeling experiments were used for the mechanistic investigation. Based on a pivotal point in the mechanistic understanding of the action of t-butoxide anion, several uncovering reactions such as direct access to value-added chiral α-amino acid t-butyl ester with almost complete retention of optical purity via elaborative transesterification, non-hydrolytic deesterification of esters, and selective bond cleavage of 3-benzoyloxazolidin-2-ones, were successfully achieved.


 Please wait while we load your content...
Please wait while we load your content...