Transition-metal-free catalytic hydroboration reduction of amides to amines†
Abstract
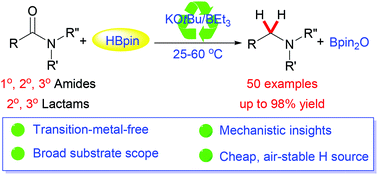
The selective catalytic reduction of amides to value-added amine products is a desirable but challenging transformation. Here, we present a combined KOtBu/BEt3 catalyst for the deoxygenative reduction of tertiary, secondary, and even notoriously challenging primary carboxamides with pinacolborane (HBpin) at 25–60 °C. This novel transition-metal-free methodology is readily applicable to the synthesis of drug molecules.


 Please wait while we load your content...
Please wait while we load your content...