Transition-metal-free radical relay cyclization of vinyl azides with 1,4-dihydropyridines involving a 1,5-hydrogen-atom transfer: access to α-tetralone scaffolds†
Abstract
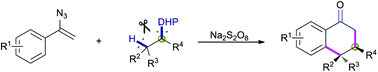
The remote C(sp3)–H functionalization enabled by a radical-mediated 1,5-hydrogen-atom transfer (HAT) process using vinyl azides and 1,4-dihydropyridines as precursors has been described. In this study, 1,4-dihydropyridines can function as 1,2-diradical synthons through sequential homolytic cleavage of an ipso-C–C bond and a β-C(sp3)–H bond. This reaction offers facile access to a diverse range of α-tetralones with excellent stereoselectivity. The utility of the present method is further highlighted by its application to rapid assembly of the tetracyclic scaffold present in furanosteroids as well as the synthesis of aromatic amines.


 Please wait while we load your content...
Please wait while we load your content...