Palladium-catalyzed reductive cross-coupling between α-bromo carboxamides and terminal alkynes†
Abstract
An efficient Pd-catalyzed reductive cross-coupling between α-bromo carboxamides and terminal alkynes was developed. Instead of affording the Sonogashira coupling products, the protocol provided a series of β,γ-alkenyl carboxamides which were subsequently subjected to catalytic hydrogenation to give the corresponding α,α-dialkyl carboxamides. A preliminary mechanistic study revealed a radical coupling process and the solvent served as the hydrogen source. The judicious selection of a base, Pd catalyst and solvent was critical for the success of the reaction.
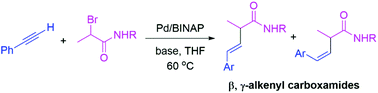


 Please wait while we load your content...
Please wait while we load your content...