A practical ortho-acylation of aryl iodides enabled by moisture-insensitive activated esters via palladium/norbornene catalysis†
Abstract
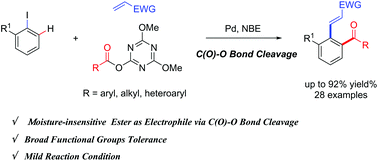
A novel ortho-arene C–H acylation of aryl iodides catalyzed by the palladium/norbornene cooperative system by employing triazine esters has been developed, leading to the synthesis of polysubstituted aryl ketones. This ortho-acylation even proceeds smoothly in water. Preliminary mechanistic experiments indicate that the strong electron-withdrawing ability and coordination chemistry of triazine play crucial roles in this transformation.


 Please wait while we load your content...
Please wait while we load your content...