C–O/C–S difunctionalized benzene derivatives via multicomponent coupling of tetraynes†
Abstract
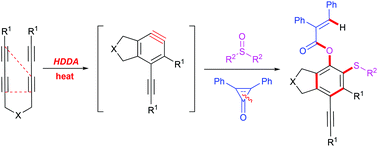
C–O/C–S difunctionalization of fused highly substituted benzene derivatives was conducted via the multicomponent coupling reaction of tetraynes, sulfoxides, and cyclopropenones. This reaction is associated with several bond cleavage and formation reactions in one pot, and also features exquisite regioselectivity and excellent yields. Preliminary mechanistic studies reveal that the reaction possibly proceeds in a sequential [2 + 2] cycloaddition, ring-opening of the cyclopropenone, protonation and dealkylation pathway.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...