Highly enantioselective 1,6-addition of dienolates to coumarins and chromones through N-heterocyclic carbene catalysis†
Abstract
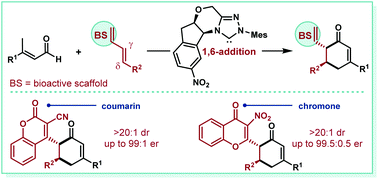
Coumarins and chromones are privileged motifs in natural products and pharmaceuticals; yet, the challenge of devising an asymmetric strategy for their δ-functionalization has remained unsolved to date. In the past three years, 1,6-addition provided new possibilities for N-heterocyclic carbene (NHC) catalyzed asymmetric carbon–carbon bond formation. However, the corresponding formal [4 + 2] cycloadditions via 1,6-addition of dienolates are far less developed. We demonstrate herein the first γ,δ-difunctionalization of cyanocoumarins and nitrochromones via NHC-catalyzed 1,6-addition of dienolates. This strategy allows the synthesis of two valuable classes of compounds in high stereoselectivities with a broad scope.


 Please wait while we load your content...
Please wait while we load your content...