How does the nickel catalyst control the doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles? The rebound mechanism†
Abstract
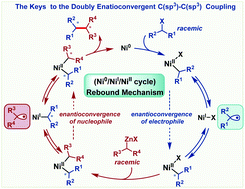
A DFT mechanistic study was performed to account for the nickel-catalyzed doubly enantioconvergent C(sp3)–C(sp3) coupling of racemic alkyl nucleophiles and electrophiles. After generating an active Ni0 species, the coupling proceeds via the Ni0/NiII catalytic cycle, but the NiII species can undergo a rebound process using NiI as the transient species for erasing and resetting the chiralities of the substrates. Reductive elimination is the stereoselectivity-determining step, thus enabling the ligand to control the stereoselectivity by favoring the one leading to the major (S)(S)-product through the steric effect among the four stereoisomers of the reductive elimination transition state. Expectedly, the disclosure of the rebound mechanism offers an inspiration for developing doubly enantioconvergent C(sp3)–C(sp3) couplings, as well as general enantioconvergent couplings.


 Please wait while we load your content...
Please wait while we load your content...