Organocatalytic site- and stereoselective 1,6-additions of N-aryl-3-oxobutanamides to propargylic aza-p-quinone methides†
Abstract
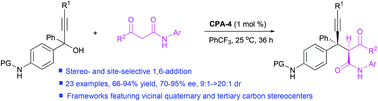
A chiral phosphoric acid catalyzed site-selective 1,6-conjugate addition of N-aryl-3-oxobutanamides to in situ formed propargylic aza-p-quinone methides from propargylic alcohols has been established and this represents the first report on organocatalytic site- and stereoselective 1,6-conjugate addition of propargylic aza-para-quinone methides. Furthermore, the synthetic strategy enables the formation of diarylmethyl alkynes bearing vicinal quaternary and tertiary carbon stereocenters in high yields with high asymmetric induction.


 Please wait while we load your content...
Please wait while we load your content...