Ambient access to a new family of pyrrole-fused pyrazine nitrones via 2-carbonyl-N-allenylpyrroles†
Abstract
The chemo-, regio- and stereoselective synthesis of pyrrole-fused pyrazine nitrones via the direct reaction of 2-carbonyl-N-allenylpyrroles (readily accessible from the corresponding NH-pyrroles) with hydroxyl amine hydrochloride has been developed. This is the first example of N-oxide formation from oxime and allene (towards the sp-carbon atom). The reaction proceeds in a one-pot manner under mild conditions without metal catalysts and tolerates all the substituents. The cyclization directions with the participation of terminal and internal allenyl carbon atoms have been compared using quantum chemical methods. The use of available feedstock, high yields of the products and selectivity of the process create a platform to access a new class of pyrazine nitrones having potential for the design of compounds with tailor-made properties.
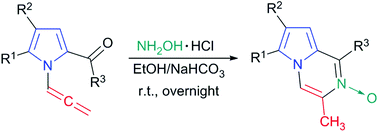


 Please wait while we load your content...
Please wait while we load your content...