From a 1,2-azaborinine to large BN-PAHs via electrophilic cyclization: synthesis, characterization and promising optical properties†
Abstract
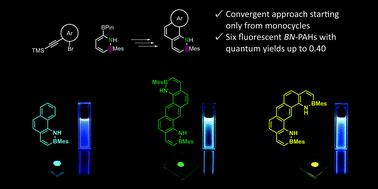
We present a convergent synthetic route towards boron–nitrogen containing polycyclic aromatic hydrocarbons (BN-PAHs) that allowed us to synthesize six derivatives. Starting from the conjunction of a 1,2-azaborinine nucleophile and various aryl electrophiles, the key step was the extension of the aromatic system via an electrophilic ring closure of the respective alkyne precursors. Our route allows the use of substituted PAH precursors to be circumvented, which are often unavailable. Instead, it builds up the BN-PAHs solely from easily accessible monocycles. All derivatives were emissive in solution and solid state with quantum yields up to Φlum = 0.40 and small Stokes shifts. The emission wavelengths in solid state were notably dependent on the connectivity of the rings. Due to excimer formation in one derivative, its emission was significantly redshifted with a comparatively slow secondary photoluminescence (PL) decay.


 Please wait while we load your content...
Please wait while we load your content...