Identification, cloning, expression and functional interrogation of the biosynthetic pathway of the polychlorinated triphenyls ambigol A–C from Fischerella ambigua 108b†
Abstract
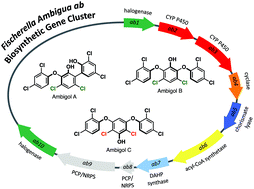
The terrestrial cyanobacterium Fischerella ambigua 108b produces the three polychlorinated triphenyls ambigol A–C that exhibit interesting antimicrobial, antiviral and cytotoxic activities. They are structurally related to polybrominated diphenylethers synthesized by diverse marine bacteria that are known to be highly toxic and are bioaccumulating in natural food webs. All ambigols display unusual connectivities: Ambigols A and B exhibit chlorination and ambigol C biaryl-ether bonds in the relative meta position at the central phenol unit, which is flanked by two 2,4-dichlorophenol units in all three compounds. Here we report on the identification of the biosynthetic gene cluster (BGC) reponsible for ambigol production in F. ambigua. After bioinformatic discovery of a putative ambigol BGC (ab) containing 10 genes, we cloned and heterologously expressed this cluster in Synechococcus elongatus PCC 7942 using Direct Pathway Cloning (DiPaC). In vivo and in vitro characterization of the two cytochrome P450 enzymes present in the ab BGC revealed complementary selectivity for either biaryl-ether bond (Ab2) or biaryl formation (Ab3) and provided a biosynthetic route to the ambigols.
- This article is part of the themed collection: Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...