Asymmetric catalytic construction of fully substituted carbon stereocenters using acyclic α-branched β-ketocarbonyls: the “Methyl Rule” widely exists
Abstract
The catalytic asymmetric construction of tetrasubstituted carbon stereocenters constitutes one of the most challenging research topics in organic synthesis, which further plays vital roles in diverse fields including medicinal and materials chemistry. In this context, the α-functionalization of β-ketocarbonyl compounds serves as one of the most frequently used strategies to address the issue. Compared to cyclic β-ketocarbonyls, the catalytic asymmetric α-functionalization of acyclic β-ketocarbonyls is more difficult but has gained enough progress over the last several decades. This review illustrates the recent advances in this field, including asymmetric fluorination with acyclic α-branched β-ketocarbonyl participation, Michael addition, amination, aldol reaction, α-alkylation, α-alkynylation, α-oxygenation, Mannich reaction, etc. Furthermore, a thorough survey of all these reactions indicates the existence of a general principle which is called the “Methyl Rule”. Alternative methods that can complement the deficiency of reports of the use of direct asymmetric catalysis are also presented.
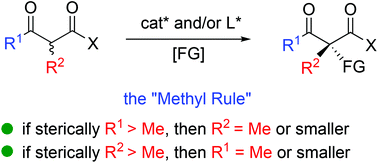
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers Review-type Articles


 Please wait while we load your content...
Please wait while we load your content...