(2-Ketulosonyl)onate 2,3-O-thionocarbonate donors for the synthesis of KO and KDO α-glycosides and a one-pot glycosylation method for 2-keto acid donors†
Abstract
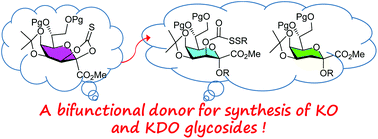
We report a short synthetic strategy for direct preparation of (2-ketulosonyl)onate donors. These donors feature a cyclic thionocarbonate group at the C2 and C3 positions, which plays a multitude of chemical roles. It protects the C2 and C3 hydroxyls during building block preparation. In glycosylation, the thionocarbonate can be activated by a sulfenylating promoter and serves as a leaving group. Because of its β-configuration, it also plays the role of a stereo-directing group during the formation of the α-glycosidic bond. In addition, the thionocarbonate donors can couple with thioglycoside acceptors. This orthogonal property was used for the development of the first one-pot glycosylation method for 2-keto acid sugars.
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...