Synthesis of aziridines with multiple chiral substitutions by copper-catalyzed diastereoselective radical aminotrifluoromethylation of alkenes†
Abstract
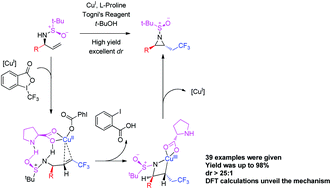
A one-step catalytic and diastereoselective method for the synthesis of aziridines possessing multiple chiral substitutions by radical aminotrifluoromethylation of alkenes has been developed for the first time. The reaction utilizes a Cu(I)/L-proline complex as a catalyst and a chiral sulfinamide group performs the role of nucleophile and chiral directing group. This synthetic strategy provides one-step access to a wide variety of substituted aziridines with good chemical yield, excellent diastereoselectivity and broad functional group tolerance. A possible reaction mechanism is proposed based on DFT calculations. The method should be useful for the rapid synthesis of chiral CF3-containing building blocks and novel drug molecules.


 Please wait while we load your content...
Please wait while we load your content...