DFT study on the E-stereoselective reductive A3-coupling reaction of terminal alkynes with aldehydes and 3-pyrroline†
Abstract
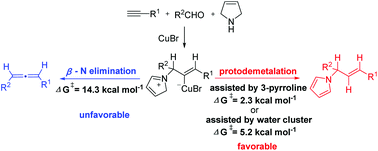
The mechanism of the Cu(I)-catalyzed reductive A3-coupling reaction of terminal alkynes with aldehydes and 3-pyrroline for the synthesis of E-allylic amines has been studied by DFT calculations. The calculations suggest that the pathway selectivity is determined by the activation energy difference between β-N elimination and protodemetalation from the iminium intermediate (INT7) formed via the rate-limiting 1,5-H transfer of the CuBr-coordinated propargylic amine (INT4). The stepwise intermolecular protodemetalation assisted by 3-pyrroline or water cluster proceeds more efficiently than β-N elimination, resulting in the formation of an allylic amine instead of an allene as the final product. Notably, the aromatization of the azacycle (formed from 3-pyrroline) is an essential driving force for the preference of the allylic amine products. In addition, the formation of a hydrogen bond between the hydroxyl group of the terminal alkyne and the migrating hydrogen stabilizes the transition state of the 1,5-H transfer step, thus, increasing the reaction reactivity.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...