A semipinacol rearrangement of vinylogous α-ketol cocatalyzed by a cinchona-based primary amine and N-Boc-phenylglycines: mechanisms, roles of catalysts and the origin of enantioselectivity†
Abstract
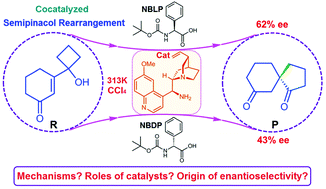
Spirocyclic diketones with chiral all-carbon quaternary stereocenters constructed via a semipinacol rearrangement of vinylogous α-ketol cocatalyzed by a cinchona-based primary amine and Brønsted acids such as N-Boc-phenylglycine can be obtained with good enantiocontrol. Two different catalytic systems including cinchona-based primary amine/N-Boc-L-phenylglycine and cinchona-based primary amine/N-Boc-D-phenylglycine can lead to the same major products but with different enantioselectivities. To uncover the detailed reaction mechanism of the semipinacol rearrangement, the roles of catalysts and the origin of enantioselectivity, quantum mechanical calculations were performed. The reaction pathways investigated show that the reactions proceed via the steps of complexation, nucleophilic addition, dehydration, carbon atom migration, enamine–imine tautomerization, imine hydrolysis, Walden inversion and catalyst regeneration. Analyses of noncovalent interactions and quantum theory of atoms in molecules were employed to elucidate the intermolecular interactions occurring between the catalysts and reactants and their roles. The distortion/interaction models were used to reveal the origin of enantioselectivity by analyzing the transition state in the enantioselectivity-determining step of carbon atom migration. The results show that the cinchona-based primary amine plays a crucial role in determining the enantioselectivity while the Brønsted acid additives influence the enantioselectivity to some extent. It is expected that the results would shed light on the organocatalytic semipinacol rearrangement, the roles of the co-catalytic system and the origin of enantioselectivity.


 Please wait while we load your content...
Please wait while we load your content...