BODIPY-amino acid conjugates – tuning the optical response with a meso-heteroatom†
Abstract
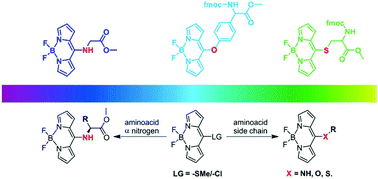
The presence of a heteroatom at the meso-position of BODIPY significantly influences the π-cloud of the main chromophore, modifying the final optical properties. In this work we obtained a series of amino acid-BODIPY hybrid skeletons with good yields through a nucleophilic aromatic substitution reaction. The heteroatom came from amino acid chains where the specific location of nitrogen, sulfur or oxygen opened a possibility of formation of a family of specific derivatives. The obtained series of hybrid materials were investigated with different spectroscopic techniques, giving insights into the specific behaviour shown by the derivatives. The observed optical responses were demonstrated to be dependent on the type of the heteroatom attached to the meso-position of the BODIPY skeleton, substantially modulating the optical response depending on the type of C–X (X = N, S, and O) interaction. Moreover, the influence of the heteroatom connected to the meso-position showed an interesting correlation in the NMR experiments (1H and 13C) and substantial differences were observed in the theoretical calculation models (DFT).


 Please wait while we load your content...
Please wait while we load your content...