Chiral strong Brønsted acid-catalyzed enantioselective addition reaction of simple olefins with ethyl glyoxylate†
Abstract
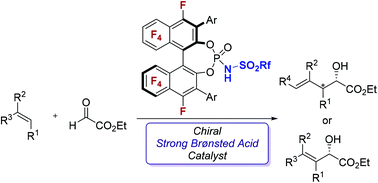
An enantioselective intermolecular carbonyl–ene reaction and a Friedel–Crafts-type reaction of 1,1,2-trisubstituted and 1,2-disubstituted simple olefins with ethyl glyoxylate were developed using F10BINOL-derived N-sulfonyl phosphoramide as the chiral strong Brønsted acid catalyst. The present reactions feature an atom-economical and environmentally benign approach to the synthesis of enantioenriched allylic and homoallylic alcohols.


 Please wait while we load your content...
Please wait while we load your content...