Silver(i)-catalyzed selective hydroalkoxylation of C2-alkynyl quinazolinones to synthesize quinazolinone-fused eight-membered N,O-heterocycles†
Abstract
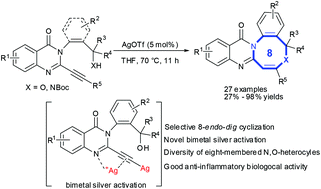
We report a silver-catalyzed hydroalkoxylation of C2-alkynyl quinazolinones to prepare a series of novel quinazolinone-fused eight-membered N,O-heterocycles in good-to-excellent yields through a selective 8-endo-dig cyclization. Mechanistic studies revealed that the silver catalyst might aid bidentate coordination of an imine group and alkyne to facilitate 8-endo-dig cyclization to afford eight-membered N,O-heterocycles. Also, the proposed bimetal silver intermediates might promote hydroalkoxylation rapidly for quinazolinones bearing terminal alkynes at the C2-position. Biological evaluations revealed that most of the designed quinazolinone-fused eight-membered N,O-heterocycles inhibited nitric-oxide generation significantly in lipopolysaccharide-stimulated RAW264.7 cells and displayed their bioactivity as potentially good anti-inflammatory agents.


 Please wait while we load your content...
Please wait while we load your content...