A metal-free synthesis of 1,1-diphenylvinylsulfides with thiols via thioetherification under continuous-flow conditions†
Abstract
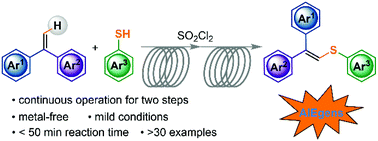
Aggregation-induced emission (AIE) compounds have emerged as an important type of material in a variety of high-tech applications. 1,1-Diphenylvinylsulfides represent a new class of AIE luminogens following the restriction of intramolecular motion (RIM) mechanism. However, synthetic approaches that allow efficient access to such molecules are rather limited owing to the steric hindrance in the multi-aryl substituted ethene skeleton. Herein, we report a new and metal-free C–S bond construction strategy to access 1,1-diphenylvinylsulfides via thioetherification of 1,1-diphenylethenes with thiols under mild conditions. Moreover, the isolated yields were found to be dramatically improved by streamlining the synthesis using a simple continuous-flow setup. With the combined advantages of the new C–H sulfenylation protocol and continuous-flow chemistry, various 1,1-diphenylvinylsulfides were generated in good yields in a less than 50 min residence time without the use of metal catalysts from all easily accessible materials, and a variety of derivatives were easily afforded via post-modifications.


 Please wait while we load your content...
Please wait while we load your content...