Straight access to highly fluorescent angular indolocarbazoles via merging Au- and Mo-catalysis†
Abstract
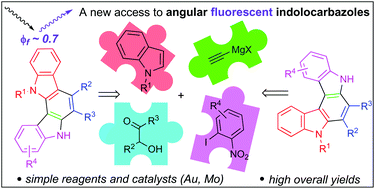
A straightforward and efficient synthesis of the two less explored types of indolocarbazoles has been developed. Two different processes for the carbazole nucleus preparation, a gold-catalysed regioselective cyclization followed by the dioxomolybdenum-catalysed version of Cadogan reductive cyclization, enables the sequential construction of two carbazole cores. The procedure features total regioselectivity and high overall yields. The required starting α-indol-3-ylalkyl propargylic alcohols are easily and efficiently accessed from commercially available reagents. In addition, the photoluminescent properties of two indolo[2,3-c]carbazoles, with fluorescence quantum yields around 0.7, have been studied.


 Please wait while we load your content...
Please wait while we load your content...