The protecting group enabled para-selective C–H benzylation of anilides via iron(ii) catalysis: a convenient approach for the synthesis of triarylmethanes†
Abstract
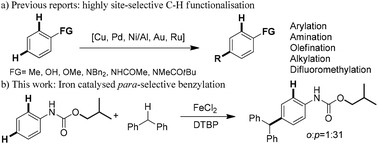
We report the para-selective C–H benzylation of phenyl carbamate with an iron(II) catalyst. This new method provides an efficient and convenient approach for the synthesis of triarylmethanes starting from readily available anilines and diphenylmethane. Preliminary mechanistic studies revealed that the iron(II) catalyst was essential and the use of the sterically demanding isobutoxy group led to high para-selectivity.


 Please wait while we load your content...
Please wait while we load your content...