A vinylogous Michael reaction of 2-furanone dimers with α,β-unsaturated nitroolefins for constructing chiral γ,γ-disubstituted butenolides†
Abstract
An efficient bifunctional thiourea-catalyzed asymmetric vinylogous Michael addition of 2-furanone dimers to α,β-unsaturated nitroolefins has been developed to afford functional chiral γ,γ-disubstituted butenolides in good yields (up to 95%) with good to excellent stereoselectivity (up to >20 : 1 dr, 99% ee). DFT calculations were carried out to explain the high stereoselectivity.
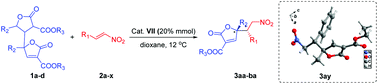


 Please wait while we load your content...
Please wait while we load your content...