Ligand-controlled palladium catalysis enables switch between mono- and di-arylation of primary aromatic amines with 2-halobenzothiazoles†
Abstract
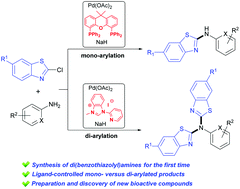
Herein, a wide range of mono- and di-arylated products were efficiently prepared from C–N coupling of 2-halobenzothiazoles and primary aromatic amines, and representative compounds 3b and 4b were further confirmed by X-ray crystallography. It was noteworthy that the di-arylated products, denoted as di(benzothiazolyl)amines, are new chemical entities which have not yet been reported. Moreover, the ligand-controlled protocol was extended to the synthesis of target molecules bearing a diphenyl ether or diphenyl amine scaffold. To our delight, several compounds exhibited good inhibitory activity against succinate-cytochrome c reductase (SCR), which revealed the practical application of this protocol. Notably, selective mono- and di-arylation could be switched simply by varying the ligand from Xantphos to a pyridine-functionalized N-heterocyclic carbene (NHC) ligand, and further investigations were carried out to elucidate the possible reason for this new finding.


 Please wait while we load your content...
Please wait while we load your content...