TFA/TBHP-promoted oxidative cyclisation for the construction of tetracyclic quinazolinones and rutaecarpine†
Abstract
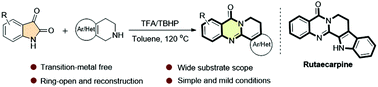
An efficient TFA/TBHP-promoted oxidative cyclisation of readily available isatins with 1,2,3,4-tetrahydroisoquinolines has been firstly developed. This protocol features simple operation, metal-free conditions and broad substrate scope. Under mild conditions, a wide range of tetracyclic quinazolinones were obtained in a highly efficient manner. Moreover, the potential utility of this strategy was demonstrated by one-step synthesis of a natural alkaloid Rutaecarpin.


 Please wait while we load your content...
Please wait while we load your content...