Ag2O-catalysed nucleophilic isocyanation: selective formation of less-stable benzylic isonitriles†
Abstract
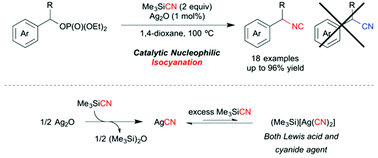
Silver-catalysed benzylic isocyanation was achieved. When a readily available silver(I) compound, Ag2O, was employed as a catalyst in 1,4-dioxane, nucleophilic substitution of benzylic phosphates with trimethylsilyl cyanide (TMSCN) gave the less-stable benzylic isonitriles exclusively. No benzylic nitrile, which is the more stable regioisomer, was observed through the reaction. Notably, secondary benzylic isonitriles, which had not been available by catalytic substitutions, as well as primary ones were obtained in high yield. The selection of the leaving group of the benzylic electrophiles was crucially important, namely, the desired isonitriles were obtained in high yield only when the phosphates were used as substrates. A mechanistic study suggested that (TMS)[Ag(CN)2] formed in situ from the silver(I) salt and excess amounts of TMSCN functioned as an active species, and the substitution proceeded through an SN1-type process rather than in SN2 fashion.


 Please wait while we load your content...
Please wait while we load your content...