Access to SCN-containing thiazolines via electrochemical regioselective thiocyanothiocyclization of N-allylthioamides†
Abstract
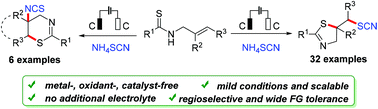
An electrochemical thiocyanothiocyclization of N-allylthioamides has been developed for the synthesis of SCN-containing 2-thiazolines. This method provides a green and efficient approach to generate 5-exo-cyclization 2-thiazolines with a broad substrate scope and good yields. In addition, 6-endo-cyclization isothiocyanato thiazines are formed regioselectively when cyclic thioamides are used as reactants. The reaction is easy to proceed under catalyst-, additive- and oxidant-free conditions.
- This article is part of the themed collection: 2020 Organic Chemistry Frontiers HOT articles


 Please wait while we load your content...
Please wait while we load your content...