An Aspidosperma-type alkaloid dimer from Tabernaemontana bovina as a candidate for the inhibition of microglial activation†
Abstract
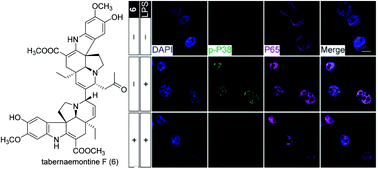
Twelve undescribed bisindole alkaloids, tabernaemontines A–L, were simultaneously isolated from the leaves of Tabernaemontana bovina and their structures were elucidated by spectroscopic methods. All alkaloids consist of two Aspidosperma-type units with various intramolecular linkages. Particularly, tabernaemontines A–D were assembled through C-3/14′ with a large angle strain, whilst tabernaemontines E–K adopted a preferential conformation. Tabernaemontine L was assembled via a C-14/10′ connection. Tabernaemontines F, K and L showed good inhibition of microglial activation. As a representative of Aspidosperma-type dimers, the P38 MAPK activation inhibition properties of tabernaemontine F were tested. The promising results revealed this compound as a potential candidate for the treatment of chronic neurodegenerative diseases.


 Please wait while we load your content...
Please wait while we load your content...