Access to cyano-substituted pyrazolines through copper-catalyzed cascade cyanation/cyclization of unactivated olefins†
Abstract
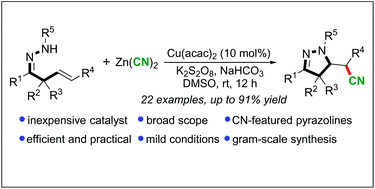
A novel and efficient copper-catalyzed domino cyanation/cyclization of hydrazone-tethered unactivated olefins has been developed. A range of cyano-containing pyrazolines have been easily obtained in good yields under mild conditions. This method provides a facile, practical, and rapid access to valuable pyrazolines with the important cyano functionality, and it is amenable for gram scale synthesis. The simultaneous formation of C(sp3)–CN and C–N bonds can be achieved in a single step.


 Please wait while we load your content...
Please wait while we load your content...