Turn-on fluorescence sensors based on dynamic intramolecular N→B-coordination†
Abstract
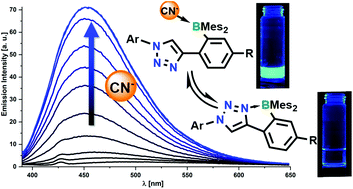
A series of ten aryl-triazole-functionalized boranes bearing BMes2-groups and capable of forming intramolecular five-membered N→B-coordinated heterocycles, has been prepared by 1,3-dipolar cycloaddition. Electronically diverse substituents ranging from 4-NMe2-phenyl (4-NMe2) and 4-OMe-phenyl (4-OMe) to 4-cyanophenyl (4-CN), 4-nitrophenyl (4-NO2) and pentafluorophenyl (C6F5) have been introduced in the periphery of the compounds to systematically vary their electronic properties and the strength of the N→B-coordination. Experimental and computational studies show that the boranes exist in dynamic equilibrium between a closed N→B-coordinated conformation and two types of non-coordinated conformations (opensyn and openanti). Their structural, optical and electrochemical properties have been characterized, and their binding affinities for nucleophilic anions has been tested. Individual boranes exhibit either fluorescence quenching (4-Me, 4-OMe) or fluorescence turn-on (4-CN, 4-CF3, C6F5, 3,5-(CF3)2) upon coordination of anions. Further analyses show that the binding affinity to cyanide can be tailored through variation of the peripheral substituent, ranging from 5.79 (±0.11) for 4-Me to 6.53 (±0.15) for the 4-CN-substituted borane. These experimental data correlate with computed anion binding affinities, which indicate that the effective Lewis acidity at boron covers a range reaching from the parent compound PhBMes2 to the much more Lewis acidic Ph3B.


 Please wait while we load your content...
Please wait while we load your content...