Visible-light-mediated Barbier allylation of aldehydes and ketones via dual titanium and photoredox catalysis†
Abstract
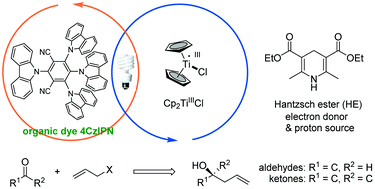
This study reports a photocatalytic Barbier-type allylation of various aldehydes and ketones with allyl halides for the synthesis of homoallylic alcohols driven by dual titanium and photoredox catalysis. This environmentally benign process uses the organic dye 2,4,5,6-(9H-carbazol-9-yl)isophthalonitrile (4CzIPN) as a photocatalyst and Hantzsch ester (HE) as an electron donor instead of stoichiometric metallic reductants.


 Please wait while we load your content...
Please wait while we load your content...