Expeditious access of chromone analogues via a Michael addition-driven multicomponent reaction†
Abstract
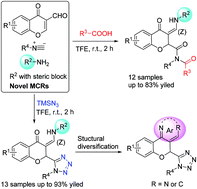
A Michael addition-driven four-component reaction (4-CR) was developed for derivatizing chromones by strategically suppressing competing 4-CR Ugi reaction without a catalyst. A series of structurally diverse 4-oxochroman-2-carboxamides was synthesized with this one-pot protocol. In addition, the new reaction was expanded for the synthesis of a series of tetrazole substituted chromones by replacing carboxylic acid with trimethylsilyl azide (TMSN3). The imine functional group and the corresponding aldehyde hydrolysed from the imine were utilized for further structural diversification.


 Please wait while we load your content...
Please wait while we load your content...