Nidulaxanthone A, a xanthone dimer with a heptacyclic 6/6/6/6/6/6/6 ring system from Aspergillus sp.-F029†
Abstract
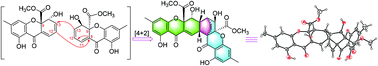
Nidulaxanthone A (1), a xanthone dimer bearing an unprecedented heptacyclic 6/6/6/6/6/6/6 system, together with a new monomeric nidulalin D (2) and four known analogues (3, 4, 5 and 6), were isolated from Aspergillus sp. F029. Biosynthetically, 1 was likely generated via [4 + 2] cycloaddition of precursor 3. Compounds 1, 2, and 3 exhibited cytotoxicity with IC50 values ranging from 3.2 to 21.9 μM, and antibacterial activity with MIC50 values of 13.2 and 19.6 μg ml−1 for 2 and 3, respectively.


 Please wait while we load your content...
Please wait while we load your content...