Synthesis and biological evaluation of suffrutines A, B and their N-fused analogues†
Abstract
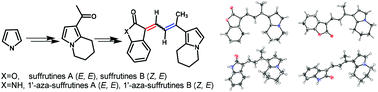
A facile synthetic route to the natural products suffrutines A (1a), B (1b) and their N-fused analogues (2a and 2b) has been accomplished starting from pyrrole in 8 steps. Their structures were elucidated by a series of NMR spectra and single crystal X-ray crystallography. The in situ1H NMR monitoring experiments showed the interconversion properties of the four isomers of suffrutines and their stability order 1a (E, E) > 1b (Z, E) > 1d (Z, Z) > 1c (E, Z) in the solution state under ambient conditions. DFT calculations further confirmed the tautomerization results. The preliminary biological test results showed that 1′-aza-suffrutines B (2b) was the most potent in cytotoxic study (MCF-7, IC50 = 7.31 μM), while 1′-aza-suffrutines A (2a) was the most active in a 14-day colony formation assay. In addition, 2a was proved to be a cancer cell apoptosis inducer in annexin V-FITC/PI dual staining tests.


 Please wait while we load your content...
Please wait while we load your content...