Divergent syntheses of spirooxindoles from oxindole-embedded four-membered synthon via cycloaddition reactions†
Abstract
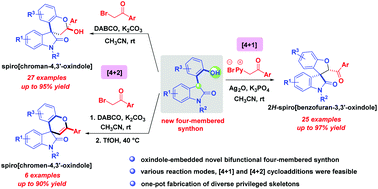
The highly efficient construction of five and six membered heterocycle fused spirooxindoles was achieved via the [4 + 1] and formal [4 + 2] cycloaddition reactions between our rationally designed four-membered synthons and pyridinium methylides and α-bromoacetophenones, respectively. A wide range of richly decorated oxindole-containing chroman, chromen, and 2H-benzofuran derivatives were synthesized in moderate to high yields and diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...