Targeted isolation of two disesquiterpenoid macrocephadiolides A and B from Ainsliaea macrocephala using a molecular networking-based dereplication strategy†
Abstract
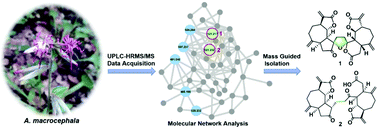
A molecular networking-based dereplication strategy was applied for the phytochemical investigation of Ainsliaea macrocephala, leading to the isolation of two novel disesquiterpenoid macrocephadiolides A (1) and B (2). Their structures, featuring a rare 5,6-spirocyclic ketal lactone core and a C-15/C-15′ linkage between a guaianolide and a 3,4-seco-guaianolide monomer, respectively, were established by extensive spectroscopic analysis and confirmed further by single-crystal X-ray crystallography. Compounds 1 and 2 showed a potent inhibitory effect on nitric oxide (NO) production, with IC50 values of 0.99 and 6.13 μM, respectively, in lipopolysaccharide (LPS)-stimulated RAW264.7 macrophages. Compound 1 dose-dependently suppressed the expression of inducible NO oxidase (iNOS) through inhibiting nuclear factor-κB (NF-κB) activation.


 Please wait while we load your content...
Please wait while we load your content...