Palladium-catalyzed cross-coupling of (hetero)aryl or alkenyl sulfonates with aryl titanium as the multi-functional reagent†
Abstract
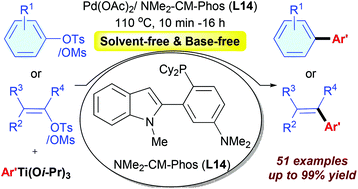
The first palladium-catalyzed cross-coupling reaction of aryl/heteroaryl and alkenyl mesylates and tosylates with aryl titanium as the multi-functional reagent is reported. Using the catalyst system of Pd(OAc)2 associated with the new NMe2-CM-Phos (L14), a broad range of electron-rich, electron-neutral, electron-deficient, and sterically hindered aryl/heteroaryl and alkenyl mesylates and tosylates are well coupled with aryl titanium reagents to give the corresponding products in good to excellent yields. The catalyst loading down to 0.2 mol% Pd and the reaction time shortening to 10 min can be achieved. The reaction can be easily scaled up to the gram scale without diminishing the product yield.


 Please wait while we load your content...
Please wait while we load your content...