Lithium amide catalyzed hydroboration of nitriles†
Abstract
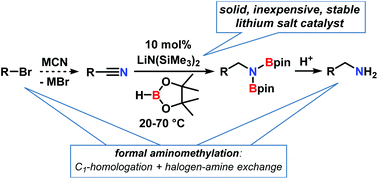
The sequential combination of the facile substitution of organohalides with the nitrile function and the hydroboration of the resultant nitriles enables a straight-forward access to synthetically versatile primary amines via a formal C1 homologation. Several catalysts have been reported for the latter transformation, mostly with transition metal catalysts (Ti, Fe, Co, Ni, Zn, Ru, Mo). Very few main group metal catalysts have been demonstrated to show equal activity. This work reports an operationally facile hydroboration of nitriles at room temperature that operates under catalysis of the solid, stable, and inexpensive salt lithium hexamethyldisilazide, LiN(SiMe3)2. The reaction displayed good tolerance of functional groups including F, Cl, Br, I, pyridyl, and thiophene substituents. Carbonyl derivatives readily reacted under the same conditions. Mechanistic studies were indicative of the catalytic role of lithium amidinate intermediates, the co-catalytic role of nitrile additives, and a reaction order of zero in borane.


 Please wait while we load your content...
Please wait while we load your content...