Copper(i)/DM-SEGPHOS-catalyzed enantio- and diastereoselective conjugate boration to α-alkylidene-γ-lactams†
Abstract
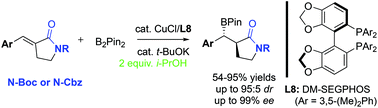
A combination of CuCl and DM-SEGPHOS catalyst system has allowed the development of highly enantioselective and diastereoselective conjugate addition of bis(pinacolato)diboron to α-alkylidene-γ-lactams. The key structural feature of conjugate acceptors, α-alkylidene-γ-lactams, was identified as the carbamate group such as N-Boc and N-Cbz, where the typical low reactivity and stereoselectivity issues associated with α,β-unsaturated lactams as conjugate acceptors were resolved.


 Please wait while we load your content...
Please wait while we load your content...