Synthesis of 3-phosphinoylbenzofurans via electrophilic phosphination cyclization†
Abstract
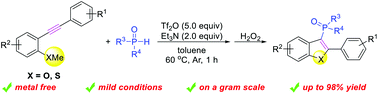
An expedient strategy for the synthesis of 3-phosphinoylbenzofurans through a cascade electrophilic phosphination cyclization has been presented. This sequential process was induced by phosphenium-cations under mild and metal-free conditions. The reaction can be scaled up to gram quantities with an excellent yield. Additionally, the obtained arylphosphine oxide product can be reduced to phosphine, which could coordinate with the transition metals ruthenium and palladium, acting as potential ligands for other important chemical reactions.


 Please wait while we load your content...
Please wait while we load your content...