Metal-free iminyl radical-mediated C–C single bond cleavage/functionalization of redox-active oxime esters†
Abstract
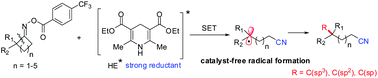
A visible-light-driven iminyl radical-mediated C–C bond cleavage and functionalization of cycloketone oxime esters have been accomplished. This protocol is simple and does not require expensive and toxic photoredox and/or transition-metal catalysis, providing a novel catalyst-free strategy for alkylation, allylation, vinylation and alkynylation through addition of C(sp3)-centered radicals to various unsaturated acceptors. The commercially available and photoactive Hantzsch ester effectively serves as an electron donor, as well as a hydrogen atom source.


 Please wait while we load your content...
Please wait while we load your content...