Visible light-mediated atom transfer radical addition to styrene: base controlled selective (phenylsulfonyl)difluoromethylation†
Abstract
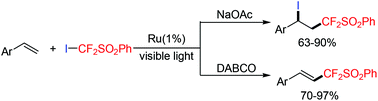
The first example of a visible light-mediated atom transfer radical addition reaction of (phenylsulfonyl)difluoromethyl iodide to styrenes has been reported. By simply tuning the organic or inorganic bases in the reaction system, both ATRA and heck-type products could be afforded with high selectivity. This method has demonstrated mild conditions, high catalytic reactivity, excellent chemoselectivity and a broad substrate scope. Besides, it offers a new solution for difunctionalization of alkenes to synthesize diverse difluoromethylated molecules after a simple work-up.


 Please wait while we load your content...
Please wait while we load your content...