(4 + 3) cycloadditions of allenyl ether-derived oxygen-stabilized oxyallyls with furans†
Abstract
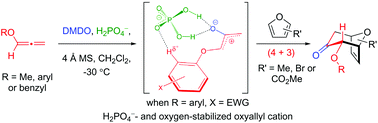
(4 + 3) cycloadditions between allenyl ethers and furans are described. The reaction features an in situ formation of oxygen-stabilized oxyallyls via epoxidations of allenyl ethers in the presence of H2PO4−. The multiple interactions between the oxygen-stabilized oxyallyl species and H2PO4− were studied using DFT calculations for rationalization of the regio- and diastereoselectivity of this cycloaddition. The utilities of this cycloaddition have been demonstrated by converting the (4 + 3) cycloadduct into the cyclohepta[b]benzofuran skeleton of frondosin B in two steps.


 Please wait while we load your content...
Please wait while we load your content...