Isolation and structure determination of two new nosiheptide-type compounds provide insights into the function of the cytochrome P450 oxygenase NocV in nocathiacin biosynthesis†
Abstract
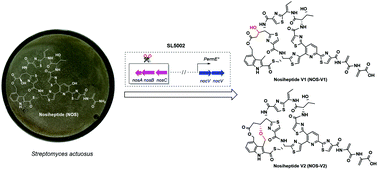
Thiopeptides, which are a class of sulfur-rich, ribosomally synthesized and post-translationally modified peptides (RiPPs), have great potential in the treatment of diseases caused by oral pathogens. Nocathiacin I (NOC-I) and nosiheptide (NOS) are two structurally similar thiopeptide members that feature an indolic side ring. In the structure of NOC-I, this side ring is further rigidified through the formation of an ether linkage; however, the related biosynthetic process remains poorly understood. Here, we report that NocV, a cytochrome P450 protein found to be unique in the biosynthetic pathway of NOC-I, is responsible for the establishment of the intramolecular ether linkage through two oxidation steps. This observation benefited from the heterologous overexpression of the gene nocV in an engineered Streptomyces strain producing the bicyclic NOS intermediate NOS1260, and subsequent isolation and structure characterization of two functionalized products. The product NOS-V1 contains a new hydroxyl group at Cα of the residue Glu6, in contrast with the other product NOS-V2, in which this hydroxyl group is further coupled with the C4 methyl group of the indolic moiety to form an ether linkage. These findings provide insights into the catalytic logic of NocV in the biosynthesis of NOC-I, during which this cytochrome P450 protein appears to act in tandem on two positions in NOS1260 by selectively hydroxylating Glu6 and then oxidatively coupling the indolic moiety. Rigidifying the side ring via ether bond formation has a positive impact on the antibacterial properties of NOS-type thiopeptides, evidenced by the improved activity of NOC-V2 against various tested oral pathogens compared with NOS1260.
- This article is part of the themed collection: Celebrating 70 Years of Shanghai Institute of Organic Chemistry


 Please wait while we load your content...
Please wait while we load your content...