Diversity-oriented approach to functional thiophene dyes by Suzuki coupling-lithiation one-pot sequences†
Abstract
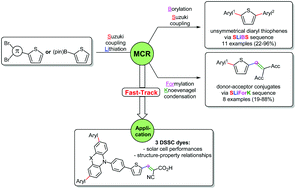
Functional thiophenes, e.g. for organic metal-free dye sensitized solar cells (DSSC), are accessible efficiently via a divergent and diversity-oriented synthetic strategy. Here, we present two straightforward, modular and comparative one-pot syntheses of diversely functionalized thiophenes with high yields starting from simple molecules, namely Suzuki-Lithiation–Formylation-Knoevenagel (SLiForK) sequence and Suzuki-Lithiation–Borylation-Suzuki (SLiBS) sequence. These methods open new avenues to interesting thiophene structure motives with potential applications as pharmaceutical active agents or in molecular electronics, which we have elucidated by a substance library of 21 diversely, mostly unsymmetrically substituted thiophenes. Finally, three novel DSSC dyes were synthesized according to the developed one-pot protocols. For illustration, DSSC devices sensitized by the selected dyes revealed promising solar cell performances, which were rationalized by photophysical and electrochemical characterization and DFT calculations.


 Please wait while we load your content...
Please wait while we load your content...