A diastereoselective approach to amino alcohols and application for divergent synthesis of dolastatin 10†
Abstract
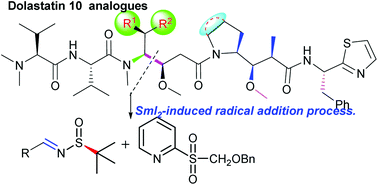
A diastereoselective approach to obtain amino alcohols 10 through SmI2-induced radical addition of chiral imine 8 with 2-(benzyloxymethylsulfonyl)pyridine 9 is described. This approach was easily used for the synthesis of non-natural amino acid 15, a flexible key fragment whose utility was demonstrated in the divergent synthesis of dolastatin 10 (1) and its nine analogues 31a, 31c, 31d, 31e, 31f, 31g, 40a, 40b and 40c were obtained.


 Please wait while we load your content...
Please wait while we load your content...